Airborne Infections Introduction
Introduction: Aerosol Transmission of Respiratory Infections
Aerosol transmission can drive respiratory infection epidemics
Respiratory infections can be transmitted by three main modes [1, 2]:
- Touching pathogens directly from an infectious person (e.g., shaking hands) or indirectly (e.g., inanimate object or fomite) followed by mucosal self-inoculation (touching eyes, nose, or mouth).
- Splashes and sprays of drops (generally >100-200 µm in diameter) containing pathogen that take ballistic trajectories from the source (e.g., drop sprays from a cough) and land on a susceptible person’s eye, nose, or mouth.
- Inhalation of aerosols (generally <100-200 µm in diameter) containing pathogens that can be released from an infected person during breathing, talking, coughing, or singing, or aerosolized from a surface or liquid, and subsequently inhaled by a susceptible person.
Although “airborne transmission” is a more general term that could refer to transmission by splashes and sprays or by aerosols, it is often used in reference to aerosol transmission. Although the relative importance of the various transmission modes is uncertain for various respiratory viruses and bacteria, this third transmission mode – via inhalation of infectious aerosols – has been implicated by epidemiologic and experimental studies as a likely driver of the population spread of influenza, measles, chickenpox, SARS, and other respiratory infections [3]. Tuberculosis, the infectious disease responsible for the largest, annual, global fatality burden can only transmit when susceptible people inhale air contaminated with infectious aerosols.
There is substantial evidence that aerosol transmission plays an important role in transmission of SARS-CoV-2 (the virus responsible for COVID-19 disease) [4-7]. The characterization of aerosol transmission forms the underpinnings of disease control measures. Engineering controls such as airflow, ventilation, filtration, and germicidal ultraviolet irradiation in indoor environments, where airborne viral levels can build up, may reduce inhalation exposure to viral aerosols [8]. Dedensification of indoor spaces [8] and the wearing of face masks can reduce indoor air contamination and inhalation exposure to infectious aerosols [9-12].
A process of airborne transmission, from infectious aerosol generation to physical and biological decay in the environment, to eventual inhalation exposure in a susceptible host, is provided by Roy and Milton [13]. Infectious aerosols can be inhaled and the sizes of the aerosols influence their deposition in the respiratory tract [2]. Respirable aerosols (≤2.5-5 µm) can reach the lower lung while thoracic (≤10-15 µm) and larger inhalable aerosols (≤100 µm) cannot. The distribution of aerosol deposition within the respiratory tree is important for transmission based on the locations of host receptors required for infection.
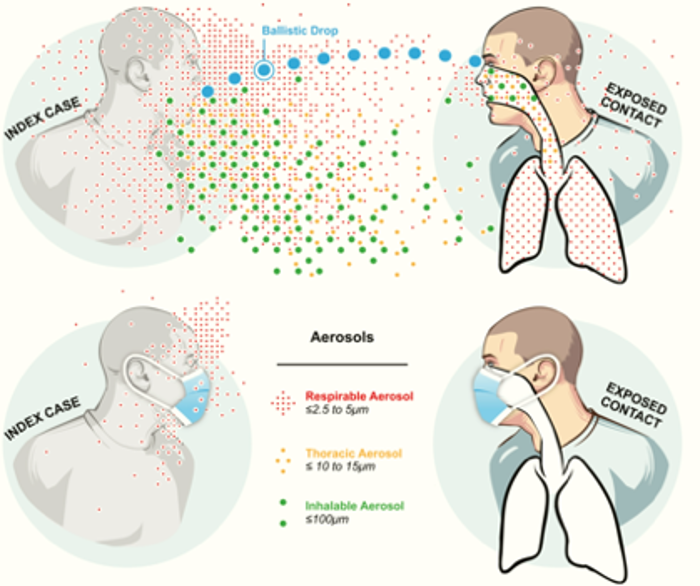
([2] Milton 2020, DOI: 10.1093/jpids/piaa079)
Disease controls efforts that reduce the concentration of infectious aerosols may succeed in limiting inhalation exposure below the infectious dose, thus interrupting transmission. In the case that control measures are not successful in stopping a transmission event, reduced exposure to infectious viral inoculum may lessen severity of disease [14]. In addition to the quantity of viral exposure, the route of exposure may also influence the resulting disease, as has been demonstrated for influenza, with severe illness initiated by low aerosol doses, and milder disease initiated by much larger doses to the upper respiratory mucosa [3, 15]. There is some evidence that influenza symptom severity may be associated with their quantity of viral shedding, although it is unclear whether infection in secondary cases initiated by the aerosol mode might lead to increased viral shedding [15]. In any case, the possibility of increased sensitivity to infection (lower viral inoculum and/or elevated severity of disease) for aerosol exposures supports precautionary measures for controlling infectious aerosols for influenza and potentially other respiratory infections.
What is an aerosol?
An aerosol is a suspension of liquid and/or solid particles within a gas (e.g., the air). In the context of respiratory infection transmission an aerosol particle may contain pathogens (generally viruses and/or bacteria) within an aqueous solution of respiratory lining fluid generated in the lung airways of an infectious person. Pathogens contained within the aerosol particle generally comprise only a small percentage of the total particle volume, with salts, proteins, and liquid comprising the majority of the volume [16, 17]. Similarly, an aerosol generated from wastewater may contain the pathogen plus some residue of the wastewater. An infectious aerosol refers to an aerosol suspension with at least some aerosol particles containing infectious virus or bacteria.
When exhaled aerosols enter the surrounding air with relative humidity <100%, as is the case for most indoor environments, they can quickly (within a few seconds or less) shrink in size due to evaporation, until achieving equilibrium with the environment [18, 19]. Evaporated particles that have reached equilibrium may contain some or no liquid and are sometimes referred to as droplet residua or droplet nuclei. These particles may be substantially smaller than their original size upon exhalation [20].
Generation of infectious aerosols
Studies quantifying viral or bacterial aerosols generated from the exhaled breath of infected humans showed higher levels of pathogens in the respirable aerosol fraction <5 µm in diameter, compared with larger aerosols [21]. Infectious influenza has been recovered from exhaled breath in the respirable size, which correlated with viral genome copy number [22]. Respirable aerosols correspond to typical sizes 1-3 µm attributed to bronchial fluid film burst in the lower lung and shear forces in the central airways [23-27] associated with breathing, talking, coughing, and sneezing. Some “speech superemitters,” for reasons that are largely unknown, can generate an order of magnitude more respiratory aerosols while talking than others [28]. Speech loudness, speech articulation, coughing, and singing have been observed to produce important increases in aerosol generation [28-30]. Heavy breathing could increase infectious aerosol generation by altering respiratory tract physical processes and through increased exhalation volume. Aerosol generating procedures in healthcare settings can lead to an increase in infectious aerosol generation and are often associated with elevated close contact exposure between healthcare workers and an infectious patient [31]. Yet, such procedures that have been classified as “aerosol generating” such as sputum induction and bronchoscopy are not required for the generation of infectious aerosols at levels that pose risk for population spread.
SARS-CoV-2 aerosols have been recovered in relatively high quantities from toilet rooms in hospital settings pointing toward the potential generation from toilet flushing [32, 33]. Viral aerosolization from apartment building wastewater stacks has been implicated epidemiologically in transmission of SARS [34, 35] and SARS-CoV-2 [36]. Vomiting is another likely source of infectious aerosols that can lead to transmission [18].
Viral/bacterial aerosol sizes and transport through the air
A number of studies have collected viral or bacterial aerosols at various sizes in environmental settings, including hospitals, schools, and public transportation. SARS-CoV-2 virus was recovered in highest concentration in aerosols 0.25-1 µm and above 2.5 µm in hospital settings [32]. Others have consistently recovered respiratory viruses in aerosol size fractions both ≤5 µm and >5 µm. Aerosols with a final size of approximately ≤5 µm can remain suspended in the air for hours, will mix with air similar to a gas, and are rapidly entrained in prevailing air following their release from the respiratory tract. Unlike larger aerosols, those ≤5 µm can penetrate the deep lung should they be inhaled. Aerosols up to 100 µm can remain suspended in air for minutes to hours, with deposition time related to humidity, temperature, and air currents [20]. They can move well beyond 2 m, their transport behavior influenced by initial force (e.g., heaviness of breathing, speech loudness, singing) and local air velocity [19]. Particles approximately >100 µm, generally described as the ballistic drops of spray/splash transmission, are likely to be driven more by their own momentum than external forces, are likely to fall to the ground quickly, and are less likely to reach a relevant exposure site (e.g., eyes, nose, mouth) of a susceptible person.
A recent modelling study estimated the volume of aerosols and drops depositing in the respiratory tract, given inhalation and exhalation velocities and deposition probabilities, after exposure to a talking or coughing primary case across a range of distances. Exposure from speech or cough generated aerosols and drops was dominated by drops >100 µm only at very close range to the source (≤0.5m away), and was otherwise dominated by aerosols [37]. Compared with longer range transmission, close range transmission <1.5-2 m represented the highest exposure to source emissions of airborne respiratory particles, and with exposure dominated by smaller aerosols rather than drops. Computational fluid dynamics using data from a chamber experiment showed that, compared with room ventilation air flow, expired air stream velocities had a greater effect on drop spray and aerosol transport [38]. But the influence of the expiratory air stream jet on subsequent aerosol inhalation exposure is mostly limited to the close range. In addition to filtering more than half of influenza laden aerosols in the respirable size aerosols ≤5 µm [12], face mask use can also help slow expired air streams, thus increasing the effect of ventilation air flow to reduce exposure between indoor occupants at closer distances. Close and long range transmission, which is more accurately described as a continuum, is described and illustrated by Yuguo Li (figure) [39]. Note that Li uses the term “droplet” specified at different sizes within the range of what has been characterized here as aerosols (<100-200 µm). Exposure to infectious aerosols can be substantial at 2m or more away from a source, especially during prolonged exposure as might be expected within a school classroom or during superspreading events [40], underscoring the importance of control measures including masking, ventilation, and air cleaning to reduce airborne exposure enough to decrease infection spread.
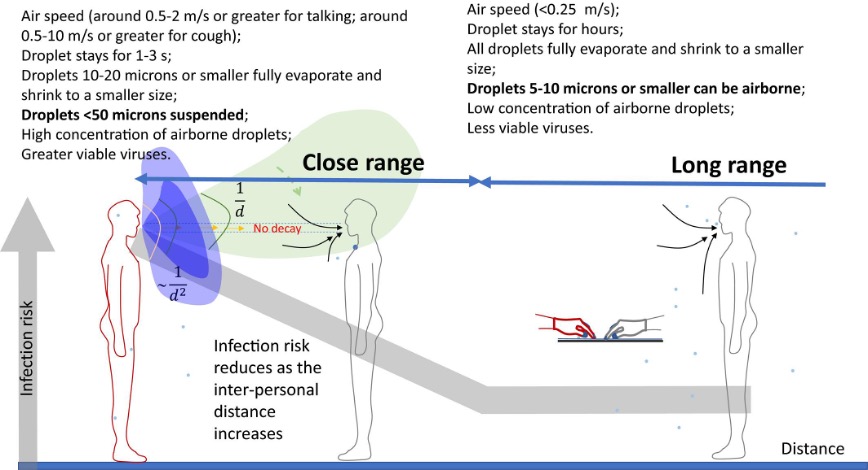
([39] Li 2021, DOI: 10.1111/ina.12806)
Efforts have been made to estimate the infectious pathogen load within aerosols and drops of different sizes (i.e., the concentration of pathogen within respiratory fluid of exhaled aerosols or drop sprays), but this has not been well characterized and the isolation of individual airborne particles to enable this analysis poses methodological challenges. Influenza viral ribonucleic acid (RNA) quantified from the exhaled breath of symptomatic cases from a university campus community (an indicator of the number of viruses present) had a geometric mean three times higher in aerosols ≤5 µm versus >5 µm [22]. RNA copies were correlated with quantitative culture, indicating that quantified RNA was reflective of infectious virus. A similar size distribution was observed from the cough aerosols of influenza cases in a separate study. 65% of influenza RNA was recovered from aerosols ≤4 µm and almost twice as much was recovered in the <1 µm compared with the 1-4 µm fraction [41]. An aerosol sampling campaign at an apartment with two influenza cases showed a predominance of infectious aerosols (by culture and qRT-PCR) in the ≤1 µm range at between 2 and 4m from a case, with some culturable virus detected >1 µm at 1.2 m away [42]. Infectious SARS-CoV-2 aerosols were recovered from VIVAS bioaerosol samplers (<0.01-10 µm size range) 2-4.8 m away from hospitalized COVID-19 cases [43]. These particle sizes correspond with the size distribution around 1 µm reported for the majority of respiratory generated aerosols during breathing [44, 45]. Sustained vocalization could increase the average generated particle size by about an order of magnitude [45]. It is unknown the extent to which the viral load increases linearly with the number of aerosols generated at various sizes. Studies that collect aerosol samples from infectious people repeatedly and with singing and vocalization across a range of loudness could address this question.
One might expect that the number of viruses contained in an aerosol particle would increase with the size of the particle if the respiratory lining fluid contains a uniform concentration of virus per unit of volume. However, laboratory-based viral aerosol generation experiments have suggested that aerosol particle volume at the submicron level may be more predictive of infectious viral number per particle than the overall aerosol size distribution [46]. It is not well characterized to what extent respiratory infections (e.g., viral replication) are evenly distributed throughout the respiratory tract and within respiratory lining fluids. A study of exhaled breath aerosols from symptomatic influenza cases showed no association between viral shedding in the upper respiratory tract and exhaled breath aerosol and no overlapping, statistically significant predictors of viral shedding [22]. This suggests distinct compartments of infection in the upper and lower respiratory tract for influenza which could influence the relationship between particle size and pathogen load.
Nonetheless, in the absence of elusive empirical evidence, some have considered a uniform concentration of SARS-CoV-2 virus in both saliva and exhaled breath aerosols to estimate transmission risk under various exposure scenarios [47, 48]. Efforts to model transmission risk and infection control effectiveness based on this assumption could result in inaccurate results, but perhaps acceptable enough to support immediate-term public health infection control strategies. Meanwhile, further research about viral load concentration across the aerosol size distribution may enhance the understanding of aerobiology, host-pathogen interaction, and transmission risk.
General guidance for SARS-CoV-2 transmission mitigation calls for distancing to help reduce close range aerosol transmission risk, as well as ventilation, filtration, and air disinfection to reduce long range aerosol transmission risk. Assurance of abundant distancing can lead to de-densifying indoor spaces which serves as a measure of source control and works synergistically with ventilation, air flow, and air cleaning strategies to deliver lower levels of pathogen-laden air to occupants. Exposure is influenced by infectious aerosol source strength, number of infectors, number of susceptibles, distance between infectors and susceptibles, time of exposure, and air flow in the room, sources of viral removal or decay through ventilation, filtration, or disinfection (e.g., germicidal UV). Given the multitude of exposure scenarios it is uncertain the extent to which close range versus long range may contribute to transmission among a population with heterogeneous susceptibility, control measures, and levels of adherence to those measures. Studies that evaluate the influence of engineering controls within buildings, assess exposure, and measure infection transmission may offer more direct evidence about the effects of mitigation strategies on the risk of infection and severe disease.
1. Li, Y., Basic routes of transmission of respiratory pathogens—A new proposal for transmission categorization based on respiratory spray, inhalation, and touch. Indoor Air, 2021. 31(1): p. 3-6. https://dx.doi.org/10.1111/ina.12786.
2. Milton, D.K., A Rosetta Stone for Understanding Infectious Drops and Aerosols. Journal of the Pediatric Infectious Diseases Society, 2020. 9(4): p. 413-415. https://dx.doi.org/10.1093/jpids/piaa079.
3. Tellier, R., et al., Recognition of aerosol transmission of infectious agents: a commentary. BMC Infectious Diseases, 2019. 19(1). https://dx.doi.org/10.1186/s12879-019-3707-y.
4. Prather, K.A., et al., Airborne transmission of SARS-CoV-2. Science, 2020. 370(6514): p. 303.2-304. https://dx.doi.org/10.1126/science.abf0521.
5. Samet, J.M., et al., Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): What We Know. Clinical Infectious Diseases, 2021. https://dx.doi.org/10.1093/cid/ciab039.
6. Greenhalgh, T., et al., Ten scientific reasons in support of airborne transmission of SARS-CoV-2. The Lancet, 2021. 0(0). https://dx.doi.org/10.1016/S0140-6736(21)00869-2.
7. Tang, J.W., et al., Covid-19 has redefined airborne transmission. BMJ, 2021. 373: p. n913. https://dx.doi.org/10.1136/bmj.n913.
8. Morawska, L., et al., How can airborne transmission of COVID-19 indoors be minimised? Environment International, 2020. 142: p. 105832. https://dx.doi.org/10.1016/j.envint.2020.105832.
9. Gandhi, M. and L.C. Marr, Uniting Infectious Disease and Physical Science Principles on the Importance of Face Masks for COVID-19. Med, 2021. 2(1): p. 29-32. https://dx.doi.org/10.1016/j.medj.2020.12.008.
10. Brooks, J.T., et al., Maximizing Fit for Cloth and Medical Procedure Masks to Improve Performance and Reduce SARS-CoV-2 Transmission and Exposure, 2021. MMWR. Morbidity and Mortality Weekly Report, 2021. 70(7): p. 254-257. https://dx.doi.org/10.15585/mmwr.mm7007e1.
11. Leung, N.H.L., et al., Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature Medicine, 2020. 26(5): p. 676-680. https://dx.doi.org/10.1038/s41591-020-0843-2.
12. Milton, D.K., et al., Influenza Virus Aerosols in Human Exhaled Breath: Particle Size, Culturability, and Effect of Surgical Masks. PLoS Pathogens, 2013. 9(3): p. e1003205. https://dx.doi.org/10.1371/journal.ppat.1003205.
13. Roy, C.J. and D.K. Milton, Airborne Transmission of Communicable Infection — The Elusive Pathway. New England Journal of Medicine, 2004. 350(17): p. 1710-1712. https://dx.doi.org/10.1056/nejmp048051.
14. Gandhi, M. and G.W. Rutherford, Facial Masking for Covid-19 — Potential for “Variolation” as We Await a Vaccine. New England Journal of Medicine, 2020. 383(18): p. e101. https://dx.doi.org/10.1056/nejmp2026913.
15. Bueno De Mesquita, P.J., et al., Influenza A (H3) illness and viral aerosol shedding from symptomatic naturally infected and experimentally infected cases. Influenza and Other Respiratory Viruses, 2020. 15(1): p. 154-163. https://dx.doi.org/10.1111/irv.12790.
16. Marr, L.C., et al., Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. Journal of The Royal Society Interface, 2019. 16(150): p. 20180298. https://dx.doi.org/10.1098/rsif.2018.0298.
17. National Academies of Sciences Engineering and, M., Airborne Transmission of SARS-CoV-2: Proceedings of a Workshop in Brief. 2020, The National Academies Press: Washington, DC Available from: https://doi.org/10.17226/25958.
18. Morawska, L., Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air, 2006. 16(5): p. 335-347. https://dx.doi.org/10.1111/j.1600-0668.2006.00432.x.
19. Xie, X., et al., How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air, 2007. 17(3): p. 211-25. https://dx.doi.org/10.1111/j.1600-0668.2007.00469.x.
20. Netz, R.R., Mechanisms of Airborne Infection via Evaporating and Sedimenting Droplets Produced by Speaking. The Journal of Physical Chemistry B, 2020. 124(33): p. 7093-7101. https://dx.doi.org/10.1021/acs.jpcb.0c05229.
21. Fennelly, K.P., Particle sizes of infectious aerosols: implications for infection control. The Lancet Respiratory Medicine, 2020. 8(9): p. 914-924. https://dx.doi.org/10.1016/s2213-2600(20)30323-4.
22. Yan, J., et al., Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proceedings of the National Academy of Sciences, 2018. 115(5): p. 1081-1086. https://dx.doi.org/10.1073/pnas.1716561115.
23. Almstrand, A.-C., et al., Effect of airway opening on production of exhaled particles. Journal of Applied Physiology, 2010. 108(3): p. 584-588. https://dx.doi.org/10.1152/japplphysiol.00873.2009.
24. Johnson, G.R. and L. Morawska, The Mechanism of Breath Aerosol Formation. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 2009. 22(3): p. 229-237. https://dx.doi.org/10.1089/jamp.2008.0720.
25. Johnson, G.R., et al., Modality of human expired aerosol size distributions. Journal of Aerosol Science, 2011. 42(12): p. 839-851. https://dx.doi.org/10.1016/j.jaerosci.2011.07.009.
26. Greening, N.J., et al., Small droplet emission in exhaled breath during different breathing manoeuvres: Implications for clinical lung function testing during COVID‐19. Allergy, 2021. 76(3): p. 915-917. https://dx.doi.org/10.1111/all.14596.
27. Morawska, L. and G. Buonanno, The physics of particle formation and deposition during breathing. Nature Reviews Physics, 2021. 3(5): p. 300-301. https://dx.doi.org/10.1038/s42254-021-00307-4.
28. Asadi, S., et al., Aerosol emission and superemission during human speech increase with voice loudness. Scientific Reports, 2019. 9(1). https://dx.doi.org/10.1038/s41598-019-38808-z.
30. Asadi, S., et al., Effect of voicing and articulation manner on aerosol particle emission during human speech. PLOS ONE, 2020. 15(1): p. e0227699. https://dx.doi.org/10.1371/journal.pone.0227699.
31. Klompas, M., M. Baker, and C. Rhee, What Is an Aerosol-Generating Procedure? JAMA Surgery, 2021. 156(2): p. 113. https://dx.doi.org/10.1001/jamasurg.2020.6643.
32. Liu, Y., et al., Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature, 2020. 582(7813): p. 557-560. https://dx.doi.org/10.1038/s41586-020-2271-3.
33. Ding, Z., et al., Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Science of The Total Environment, 2021. 753: p. 141710. https://dx.doi.org/10.1016/j.scitotenv.2020.141710.
34. Yu, I.T.S., et al., Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. New England Journal of Medicine, 2004. 350(17): p. 1731-1739. https://dx.doi.org/10.1056/nejmoa032867.
35. Li, Y., et al., Multi-zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air, 2005. 15(2): p. 96-111. https://dx.doi.org/10.1111/j.1600-0668.2004.00318.x.
36. Kang, M., et al., Probable Evidence of Fecal Aerosol Transmission of SARS-CoV-2 in a High-Rise Building. Ann Intern Med, 2020. 173(12): p. 974-980. https://dx.doi.org/10.7326/M20-0928.
37. Chen, W., et al., Short-range airborne route dominates exposure of respiratory infection during close contact. Building and Environment, 2020. 176: p. 106859. https://dx.doi.org/10.1016/j.buildenv.2020.106859.
38. Liu, L., et al., Short-range airborne transmission of expiratory droplets between two people. Indoor Air, 2017. 27(2): p. 452-462. https://dx.doi.org/10.1111/ina.12314.
39. Li, Y., The respiratory infection inhalation route continuum. Indoor Air, 2021. 31(2): p. 279-281. https://dx.doi.org/10.1111/ina.12806.
40. Kriegel, M., et al., Predicted Infection Risk for Aerosol Transmission of SARS-CoV-2. 2020, Occupational and Environmental Health Available from: http://medrxiv.org/lookup/doi/10.1101/2020.10.08.20209106.
41. Lindsley, W.G., et al., Measurements of airborne influenza virus in aerosol particles from human coughs. PloS One, 2010. 5(11): p. e15100. https://dx.doi.org/10.1371/journal.pone.0015100.
42. Lednicky, J.A. and J.C. Loeb, Detection and Isolation of Airborne Influenza A H3N2 Virus Using a Sioutas Personal Cascade Impactor Sampler. Influenza Research and Treatment, 2013. 2013. https://dx.doi.org/10.1155/2013/656825.
43. Lednicky, J.A., et al., Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases, 2020. 100: p. 476-482. https://dx.doi.org/10.1016/j.ijid.2020.09.025.
44. Papineni, R.S. and F.S. Rosenthal, The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects. Journal of Aerosol Medicine, 1997. 10(2): p. 105-116. https://dx.doi.org/10.1089/jam.1997.10.105.
46. Pan, M., J.A. Lednicky, and C.Y. Wu, Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology, 2019. 127(6): p. 1596-1611. https://dx.doi.org/10.1111/jam.14278.
47. Lelieveld, J., et al., Model Calculations of Aerosol Transmission and Infection Risk of COVID-19 in Indoor Environments. Int. J. Environ. Res. Public Health, 2020. 17(21). https://dx.doi.org/10.3390/ijerph17218114.
48. Buonanno, G., L. Stabile, and L. Morawska, Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environment International, 2020. 141: p. 105794. https://dx.doi.org/10.1016/j.envint.2020.105794.