Indoor Environment Controls
Overview of Indoor Environment Controls Against Airborne Infection
Exposure at room scale is important because of the potential for high concentrations of infectious aerosols, large numbers of exposed individuals, and prolonged exposure durations. Superspreading has been well-documented at this scale [1-4], and controls effective at this scale have the greatest potential to mitigate exposures and outbreaks at the community level [5-7]. Room scale airflow controls offer greater protection at longer versus shorter range, and could offer better protection against respirable aerosols (≤2.5–5 µm) that are more easily entrained in air flow than larger aerosols and sprays with greater deposition removal [8]. Transfer can be mitigated by ventilation, filtration, GUV, and air distribution to maximize control effectiveness (Figure). These approaches aim to reduce direct airflow connections between occupants, reduce well-mixed aerosol concentrations, and increase pathogen inactivation rates.
When an infected individual in a room wears a face mask, the number of infectious aerosols that reach the room air is substantially reduced and the velocity of the air flow from expirations, speech, or coughs is reduced. The aerosols that escape the face mask will mostly become entrained in thermal plumes rising upward out of the breathing zone. This protects the susceptible person at close range from exposure, and reduces the viral contamination in the room that might reach the susceptible person farther away. GUV can sanitize large volumes of air in the upper zone of the room so as not to expose skin or eyes to the lights, which could cause irritation. The ceiling fan (operated to draw air upward) can increase substantially the effectiveness of GUV systems by ensuring that contaminated air moves more directly and rapidly into the zone of sterilization in the upper room, with sterilized air returning downward into the occupied space.
There remain important research gaps in demonstrating the efficacy of individual room scale engineering controls and their effectiveness to reduce risk in population-based studies. Environmental quality and engineering controls in buildings are often not reported or their effects are not assessed in investigations of infection transmission. For example, two, high-profile population-based studies of SARS-CoV-2 transmission interventions in grad schools did not address the effects of controls like increased ventilation or air cleaner use because data on such controls was not collected [9, 10], perhaps because there were no improvements in ventilation or infectious aerosol removal. Even post-hoc ventilation assessment would be helpful for supporting risk estimation associated with poorly ventilated spaces. There is a greater availability of evidence on controls that are commonly recorded such as distancing, masking, and symptom checks.
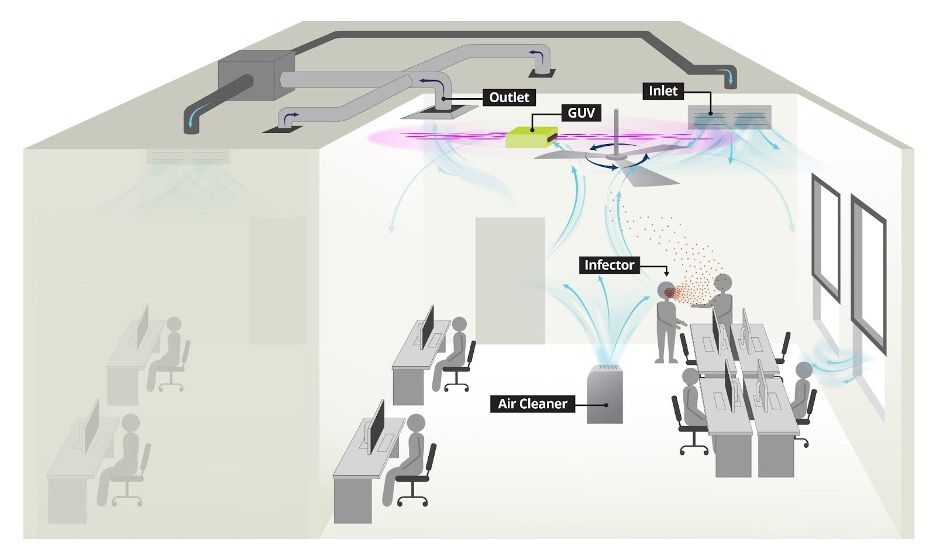
(Figure. Illustration of infectious aerosol controls in an office environment. The controls can be applied to other settings.)
1. Li, Y., et al., Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Building and Environment, 2021. 196: p. 107788. https://dx.doi.org/10.1016/j.buildenv.2021.107788.
2. Shen, Y., et al., Community Outbreak Investigation of SARS-CoV-2 Transmission Among Bus Riders in Eastern China. JAMA internal medicine, 2020. https://dx.doi.org/10.1001/jamainternmed.2020.5225.
3. Hamner, L., High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020. MMWR. Morbidity and Mortality Weekly Report, 2020. 69. https://dx.doi.org/10.15585/mmwr.mm6919e6.
4. Kriegel, M., et al., Predicted Infection Risk for Aerosol Transmission of SARS-CoV-2. 2020, Occupational and Environmental Health Available from: http://medrxiv.org/lookup/doi/10.1101/2020.10.08.20209106.
5. Bueno de Mesquita, P.J., C.J. Noakes, and D.K. Milton, Quantitative aerobiologic analysis of an influenza human challenge-transmission trial. Indoor Air, 2020. 30(6): p. 1189-1198. https://dx.doi.org/https://doi.org/10.1111/ina.12701.
6. Adam, D.C., et al., Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nature Medicine, 2020. 26(11): p. 1714-1719. https://dx.doi.org/10.1038/s41591-020-1092-0.
7. Endo, A., et al., Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Research, 2020. 5: p. 67. https://dx.doi.org/10.12688/wellcomeopenres.15842.3.
8. Li, Y., The respiratory infection inhalation route continuum. Indoor Air, 2021. 31(2): p. 279-281. https://dx.doi.org/10.1111/ina.12806.
9. Lessler, J., et al., Household COVID-19 risk and in-person schooling. Science (New York, N.Y.), 2021. https://dx.doi.org/10.1126/science.abh2939.
10. van den Berg, P., et al., Effectiveness of three versus six feet of physical distancing for controlling spread of COVID-19 among primary and secondary students and staff: A retrospective, state-wide cohort study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 2021. https://dx.doi.org/10.1093/cid/ciab230.